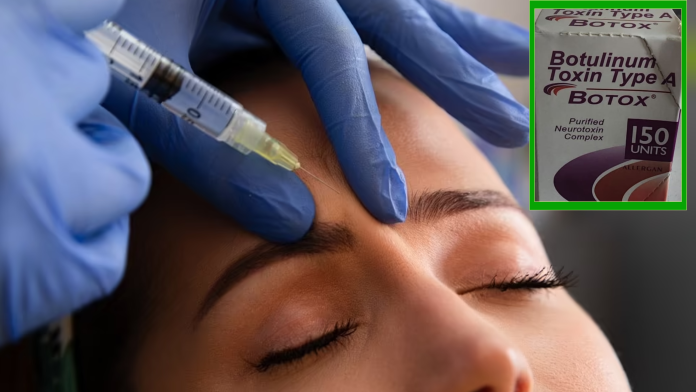
Find out why “CDC Probes Botched Botox Injections in 9 States” The Centers for Disease Control and Prevention (CDC) has initiated an investigation into a series of cases involving harmful reactions to Botox injections, which have affected at least 19 women across nine states in the United States. These reactions have been attributed to possibly fake or mishandled Botox injections, administered by individuals who were either not licensed or trained to perform the procedure, or in non-healthcare settings such as homes or spas. The states affected include Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee, and Washington. No deaths have been reported as a result of these incidents, although nine of the 19 patients were hospitalized. Four patients were treated with botulism antitoxin due to concerns that the botulinum toxin could have spread beyond the injection site.
ALSO READ: Early Lung Cancer Detection: NHS Tests New Blood Test for Drastically Improved Survival Rates
Botox, which uses a purified form of the neurotoxin botulinum toxin, is generally considered safe when administered correctly. Its primary use is to prevent or ease facial wrinkles by temporarily paralyzing the muscles. However, when injected improperly or in excessive amounts, botulinum toxin can lead to severe complications, including widespread paralysis and even death. The symptoms reported by the affected patients include blurry or double vision, drooping eyelids, difficulty swallowing or breathing, dry mouth, slurred speech, and fatigue and weakness.
The CDC’s investigation is ongoing, and it is unclear whether the reactions were due to fake products, contamination, or poor hygiene practices. The Food and Drug Administration (FDA) has also been involved in the investigation. In Illinois, health officials are investigating a case of botulism-like illness after an unlicensed provider injected a patient with what was allegedly botulinum toxin. Similarly, in Illinois, patients received injections from a nurse who was performing work outside her authority, according to the state’s Public Health Department.
The CDC initially focused its investigation on cases in Illinois and Tennessee but expanded it after receiving further reports. The patients affected by these botched Botox injections range in age from 25 to 59 years, with a median age of 39. All but one of the women were receiving Botox injections for cosmetic reasons. Medical conditions that can be treated with Botox include excessive sweating, eyelid twitching, overactive bladder, and chronic migraines. It is crucial for individuals considering Botox for medical or cosmetic reasons to ensure that their provider is licensed and trained, and that the product is FDA-approved and obtained from a reliable source.
How Patients Can Protect Themselves From Counterfeit Botox Products
To protect themselves from counterfeit Botox products, patients should take several precautions:
Verify the Provider’s Credentials: Ensure that the healthcare provider administering Botox is licensed and trained. Unlicensed or improperly trained individuals can lead to botched injections or the use of counterfeit products.
Check the Source of Botox: Patients should confirm with their healthcare provider that the Botox is purchased from an authorized source. Counterfeit Botox products can be misbranded, adulterated, contaminated, improperly stored, and transported, making them unsafe for use.
Look for Signs of Counterfeiting: Before using Botox, check the product for any signs of counterfeiting. Authentic Botox products manufactured by AbbVie display the active ingredient as “OnabotulinumtoxinA” on the outer carton and vial. The manufacturer identified on the outer carton is either “Allergan Aesthetics / An AbbVie Company” or “abbvie”.
Report Suspected Counterfeit Products: If a patient suspects they have received counterfeit Botox, they should report it to the FDA. The FDA encourages consumers to report suspected counterfeit Botox products at 800-551-3989 or through their website. Healthcare professionals and consumers can also report adverse events related to the use of any medications, including suspected counterfeit medications, to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Seek Professional Guidance: Patients should seek Botox injections from licensed professionals in medical settings. This ensures that the procedure is performed safely and that the product used is FDA-approved.
Research and Do Your Homework: Before undergoing Botox treatment, patients should research and understand the risks involved. This includes knowing who they are going to for the procedure and ensuring that the provider is reputable and experienced.
By taking these steps, patients can significantly reduce the risk of receiving counterfeit Botox products, which can lead to severe health consequences.
ALSO READ: Elderly Couple’s Bodies Found In Cornwall After Welfare Check